Sunday, November 2, 2014
Review of immunotherapy and durable benefit in melanoma!!!
This article, by some of the biggest dogs in melanoma care today, David McDermott, Celeste Lebbe`, Stephen Hodi, Michele Maio, Jeff Weber, Jedd Wolchok, John Thompson and Charles Balch, was published in Cancer Treatment Reviews, June 2014....
"Historically,...overall survival for patients with Stage IV melanoma was less than 1 year and...5-year survival rate was ~10%. Recent advances in therapy have raised 5-year survival expectations to ~20%. Notably, a subset of melanoma patients who receive immunotherapy....can achieve long-term survival of at least 5 years. A major goal...is to increase the number of patients who experience this overall survival benefit... [We] discuss...attributes of immunotherapy and newer targeted agents...how combination strategies might improve...durable benefit and long-term survival...[and] three areas we believe will be critical to making further advances...first, [we] present data from ipilimumab...trials in which a subset of patients experienced durable responses. Second, we discuss the limitations of traditional metrics used to evaluate the benefits of immunotherapies. Third, we consider emerging issues... A better understanding of these novel treatments may improve survival..., increase the number of patients who experience this...benefit, and inform the future use of these agents...."
Here's the link to read it for yourself:
Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma
Points that struck me:
Immunotherapy -
"Initial attempts to improve outcomes...in advanced melanoma focused on high-dose interleukin-2 (HD IL2), a cytokine that induces T-cell activation and proliferation. ....Phase II trial at NIH...while only 7% of melanoma patients treated with HD IL2 achieved complete regression, responses were maintained for up to 91+ months... HD IL2 may provide durable responses of over 10 years in some....use is limited by severe toxicity...[which] prompted investigations of low-dose IL2...[and though] less toxic...it has failed to produce complete and durable response. ...the experience with DC IL2 provides proof-of-concept that modulation of the immune system might offer durable...benefit... [With] more tolerable immunotherapies, the role of single agent HD IL2 remains to be determined...
Improved...understanding of tumor immunology...led to the development of targeted immunotherapies aimed at specific immune-checkpoints. [Those] currently being targeted....include cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed death-1 (PD-1), and programmed death ligand-1 (PD-L1). ...CTLA-4 acts early in the tumor response to regulate T-cell proliferation and migration...PD-1 and its ligand PD-L1 regulate T-cell activation and proliferation at the tumor site.....Ipilimumab (ipi), which targets CTLA-4, was approved by the...FDA...in 2011. [Multiple studies demonstrated survival benefit with ipi.] Data from these and other clinical trials suggest that a proportion of patients treated with ipi can achieve survival of at least 5 years. In [one study] 5-year survival was...17% for ipi 3mg/kg and 17-49% for ipi 10mg/kg.
... A meta-analysis of pooled OS data from ipi trials...[with] 1861 melanoma patients reported a 3-year OS rate of 22%...a plateau in the...Kaplan-Meier curve began at approximately 3 years after initiation of therapy, and extended through follow-up as long as 10 years...some patients...were no longer receiving treatment, suggesting that treatment-free survival is possible with ipi.
....Nivolumab and Pembrolizumab with target PD-1 have demonstrated clinical activity...whether responses will be similarly durable...remains to be determined, but preliminary evidence suggest that this may be the case.
...Phase 1...trials suggest that...targeting PD-L1 may be effective... Among 52 patients...treated with...MDX1105, 17% achieved an objective response, 27% had stable disease lasting 24 weeks or more. ...MPDL3280A, another PD-L1 inhibitor [had] objective responses in 9 of 35...patients, with all responses improving or ongoing at time of tumor assessment. A phase 1...trial is...underway with...PD-L1 inhibitor MEDI4736 in several tumor types (NCT01693562)."
Targeted therapy-
"...better understanding of biology of melanoma...led to...molecular targeted therapies....MAPK pathway is one of the major signaling networks involved in melanoma [growth]. A major driver of that pathway is BRAF...BRAF mutations are found in ~50% of melanomas...70-95% [are] V600E...[while] 5-30% are V600K...
Vemurafenib [a BRAF inhibitor]...was FDA approved in 2011 for...patients..[with] BRAF V600E... Data suggest that a subset [of these patients] may achieve survival up to 3 years with continuous, twice-daily vermurafenib treatment....but...responses...are usually of limited duration (6.7 months) due to the emergence of tumor resistance.
...In 2014 FDA approved the use of dabrafenib [a BRAF inhibitor] in combination with trametinib [a MEK inhibitor] for patients with BRAF V600E or V600K mutations....approval of combination was based on an improved ORR and median duration of response versus dabrafenib monotherapy... ORR = 76% for...combination vs 54% for...monotherapy...duration of response =10.5 months for combo vs 5.6 months for mono....
...current data suggest...Limitations with immunotherapies for some [are] low response rates, delayed onset of effect, toxicity that must be managed carefully, and [it is] difficult to predict which patients will respond...but treatment-free survival and durable responses are possible. Targeted therapies have: high response rates, side effects usually reversible with dose adjustment...but...require continuous twice daily dosing, may elicit resistance within 6-8 months, and generally do not provide long-lasting benefit after therapy is discontinued. Strategies that capitalize on the strengths and overcome the weaknesses associated with these treatments are needed and might possibly be achieved through combination and/or sequencing regimens."
Immunotherapy combination strategies-
NOTE: my shorthand synopsis -
Combination and sequenced regimens are being evaluated. Ipi and nivo: n = 17, objective response = 53%, disease control rate = 65%. Different study: n = 52, 21 had confirmed objective response ranging from 6-72+ weeks, with ongoing response observed in 91%. Immune related toxicity is greater for combo than with either single agent. Combination is being explored further.
Phase II trial ongoing with ipi and GM-CSF....prelim data...?? decreased incidence of GI and pulmonary AEs.
Ipi is being combined with BRAF inhibitors. Phase I ipi and vemurafenib was closed early due to liver toxicity. Phase II sequential ipi and vemurafenib ongoing. Ipi plus dabrafenib, with and without trametinib ongoing.
Ipi and standard cytotoxics: Ipi plus DTIC was associated with higher rates of liver problems and lower rated of gastric problems than those previously reported with either agent alone...ie toxicity is not as simple as combined effect. Ipi plus fotemustine - disease control = 46% in melanoma patients and 50% in patients with asymptomatic brain mets. Phase III trial being explored further. Ipi with temozolomide, cisplatin, interferon, or IL2 as well as ipi with carboplatin/paclitaxel is being examined.
Ipi with radiation. Various studies ongoing. Ipi with radiotherapy "has been shown to induce an abscopal effect, a phenomenon in which tumor regression occurs at a site distant from the primary site of radiotherapy." "A phase I study is investigating the maximum tolerated dose of ipi plus radiotherapy in melanoma patients with brain metastases. A phase II study is evaluating response rate associated with ipi alone vs ipi plus radiotherapy."
Characterizing long-term survivors-
"Long-term survivors among ipi-treated patients have included those who achieve CR, PR, SD, and...PD... Some patients with PD may actually develop a response or SD over time, possibly reflecting the long time required to build anti-tumor immunity... An important issue related to SD is whether this response category represents true residual disease or fibrotic tissue with no residual tumor....tumoral masses may appear to show incomplete regression, when in fact the remaining abnormal tissue is attributable to residual fibrosis....incorrect assessment of the tumor response could lead to an underestimation of the treatment effect."
"Durable responses to ipi do not appear to be associated with known prognostic factors or BRAF-mutation status. ...ipi is an effective treatment for advanced melanoma regardless of BRAF-mutation status."
[???....] "potential biomarkers that can predict response to ipi. ...absolute lymphocyte count...patients with high ALC levels after two ipi treatments had improved OS...patients with NY-ESO-1-specific CD8+ T-cell response experienced a significant survival advantage...increased circulating levels of inducible T-cells are associated with improved survival at week 7 of treatment...additional work is needed to...clarify predictive value of biomarkers in melanoma."
Refining assessment of clinical benefit-
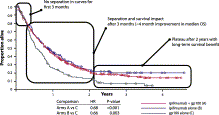
..."Nonconventional response patterns, which are unique to immunotherapy treatment include response following an initial increase in disease burden ('pseudoprogression') and response in the presence of new lesions. ... Kaplan-Meier curves from immunotherapy trials consistently show a delay in the separation of OS curves between treatment and control arms, which could relect the time needed for an immune response to translate into a survival effect. ...The challenge associated...if that it increases the statistical power necessary to differentiate the treatment and control arms."
Emerging questions for immunotherapy treatment-
"...the effectiveness of ipi in melanoma patients with brain mets continues to be explored."
"Because the subpopulation that experiences long-term benefit with ipi remains to be effectively characterized, another challenge is deciding when to move a patient who is receiving ipi on to the next therapy. ... A related challenge is determining whether immunotherapy or targeted agents should be used in the first-line setting. Prospective sequence trials will be needed to determine which treatment regimens are optimal for providing long-term benefit."
"...Patients who progress after ipi therapy may subsequently benefit from retreatment...the contribution of retreatment to overall survival remains to be determined."
So many questions remain...but we know ever so much more than when I first started this journey. Hopefully much more will be learned very soon!! Best - c
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment