Gotta say! Folks with melanoma are some of the most amazing peeps I have ever known!! I am blessed to have come to know so many incredible melanoma friends. I am very lucky to have Ellie as a dear one for some time. Not only is she a melanoma superhero, she is a talented artist, and author!!!
Ellie has certainly paid her melanoma dues, from a primary on her thigh in 1989 to abdominal mets in 2013 followed by surgeries, a zillion scans, IL-2. Yervoy, Opdivo, BRAF/MEK, side effects...AND....DRUM ROLL PLEASE ~ NED per last scans in May of 2018!!! Those results being well maintained on a Tafinlar/Mekinist combo and low dose prednisone! Way to gut it out, sister!!!
In her book,
Sketching My Way Through Metastatic Melanoma, Ellie shares her experiences through words and pictures. She made me smile, feel her worry, and recognize her world as she poignantly shares so much of what we melanoma peeps undergo. She also informs - with excellent reports on the way her therapies worked, were administered, and how she dealt with side effects.
Here are some of my favorite pics (comments are mine):
 |
| Packing is serious, yo!!! |
 |
| As is squaring our shoulders and packing away our fears! Ipilimumab/Yervoy. |
 |
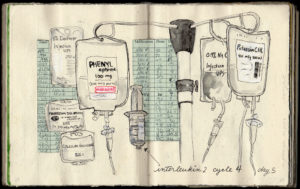
| ONO4538, MDX1106, BMS 936558, Nivolumab, Opdivo! |
 |
| The wait!!!! We've all done plenty of that one, right??? |
 |
| BRAF/MEK, targeted therapy. |
For a bit of an interview of Ellie and additional background on her work, here's a link to a write up by the AIM at Melanoma Foundation:
Sketching My Way through Metastatic Melanoma - by Eleanor Segal as well as a copy of their report below:
Sketching Her Way Through Metastatic Melanoma
Nearly every melanoma patient or survivor that AIM has met has a coping mechanism, hobby, or pastime—something they do to help them in one way or another through their cancer. The most common one we hear is connecting with other patients through social media. But others include gardening, cooking, writing, and doing yoga.
Eleanor Segal sketches. Ellie is 63, married, and a resident of Portland, Oregon. Ellie always made things, and she went to art school. She didn’t draw consistently through her life; she did metalsmithing, created lotions and potions, and worked in other media. But she began drawing again just a few years ago, and now she has sketched her way through metastatic melanoma.
But let’s go back to the beginning. In 1989, Ellie had a melanoma removed from her right thigh. All was fine until 2013, when her doctor felt something unusual during a routine pelvic exam, which turned out to be fist-sized lymph node full of melanoma. After being diagnosed with metastatic disease, she’s had four surgeries, a month of radiation, three types of immunotherapy, and currently, targeted therapy.
After her metastatic diagnosis, she joined the Portland Chapter of Urban Sketchers, a global organization. Urban sketchers always carry their gear, draw wherever they are from observation, and share their work online. The goal is not to be a perfectionist—not to spend too much time on any one sketch—but to show the world what they see, one sketch at a time.
So Ellie brought her sketchbook with her to all of her appointments, and she began sketching everything around her, from her Interleukin-2 IV fluid bags to the contents of her hospital suitcase to the view out her hospital window.
“I didn’t do this for therapy, or to help other people,” she says. “It’s for me. I make collages, write questions, make lists. Sketching allows me to observe, process, sort things, and navigate complex decisions.” Indeed, many of her sketches are combinations of items in the hospital—such as a scale or a pill—combined with details about treatment. While some patients might take notes, Ellie sketches.
It wasn’t originally a goal to make a book, but she has published a beautiful volume of watercolor sketches entitled “Sketching My Way Through Metastatic Melanoma.” Her family, friends, and healthcare providers are the lucky recipients of her books, and she has kindly included an AIM bookmark inside each volume, reminding those who receive the books to give to melanoma research.
Thank you, Ellie, for capturing in a beautiful form what so many of us have been through, and for letting AIM share a few of your sketches.
Thanks indeed, Ellie!!! While your art certainly helped you cope with all you have endured, it has become a great boon to the rest of us as well. Ubuntu!!! [I am because we are.] - love, les