A question I get often, in person, in emails, and sometimes try to answer on forums...is: What were the side effects you experienced on anti-PD1 (now Nivolumab)? So...I will try to put a clear answer together for you here.
When thinking about the side effects of any drug, you first have to think about what it is the drug is supposed to do. Then, you have to remember that no drug is a silver bullet that will address only the problem it is supposed to fix. There is always collateral damage.
Anti-PD1 (Nivolumab in my case, but Merck's product is practically equivalent) falls in the category of drugs that stimulate the immune system. Interferon is given in hopes of doing that as well. As does ipilimumab (with much better effect) and IL2 for that matter. So, what does that mean? Well, let's step back and look at the opposite drug category...drugs that suppress the immune system. I'm sure you've seen all the ads for Enbrel, Remicade, Humera, plus the original Methotrexate and even prednisone. These drugs work via different mechanisms, but their job is to DECREASE the "auto" immune response people with anything from rheumatoid arthritis, ankylosing, psoriasis, various types of colitis, and even asthma are having, and thereby minimize their symptoms and misery. SO...drugs given to stimulate the immune system with the hope of having the body attack various nasty cancer cells...melanoma in this case...can CAUSE the very symptoms that folks with autoimmune diseases have: joint pain, rashes, wheezing, colitis. Clear as mud? OK!
Well documented side effects from the data reported at ASCO and in published research papers...as experienced by ratties like myself include:
Rashes - very common. Anything from general itchiness, to red papular lesions.
Fatigue - very common. Reported frequently. Varies person to person with some folks unable to work, others just tired generally, with episodes that wax and wane.
Arthralgias - joint pain is frequently reported. Inflammation triggered by the immune response does not make joints happy.
Mucositis - irritation of the mucus membranes, from redness and tenderness to pain and lesions. (The gut is just one long tube that starts in the mouth after all....see Colitis below.)
Hypothyroidism - not as common, but certainly has happened. One lady in my study gradually lost thyroid function, and is now maintained on thyroid hormone in pill form, synthroid.
Colitis - irritation and inflammation in the bowel that can cause diarrhea and bleeding. Sometimes, causing dehydration and the need for hospitalization, fluids, discontinuation of the drug for a time or permanently, and at times prednisone to stop the progression. This is a fairly common cause of patients being taken off ipi and anti-PD1.
Pneumonitis - significant inflammation in the lungs that may be treated as noted above and has even been a cause of death for patients on ipi and anti-PD1, and obviously removal from a trial or treatment.
Pituitary failure and vision problems related to the optic nerve, as well as retinitis, have occurred but are not reported as common events.
Vitiligo - the depigmentation of the skin (and/or hair), leaving white patches. Thought to occur because of the shared antigens located on melanoma cells and normal pigment cells. It is considered a good prognostic sign that the drug is working against melanoma and occurs in 5-9% of patients on ipi or anti-PD1.
My experiences to follow.... c
Showing posts sorted by relevance for query prednisone. Sort by date Show all posts
Showing posts sorted by relevance for query prednisone. Sort by date Show all posts
Thursday, October 17, 2013
Wednesday, March 9, 2016
Immune reactions with anti-PD1 can be SERIOUS!!!!
Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. Zibelman, Pollak, Olszanski, et al. J Immunother Cancer. 2016 Feb 16.
Immune related adverse events affecting various organ systems are a recognized potential consequence of immune checkpoint inhibition. However, autoimmune inner ear disease is one complication not previously associated with the use of checkpoint inhibitors, though it has been reported after adoptive cell immunotherapy. Here we present what we believe is the first case of autoimmune inner ear disease resulting from treatment with an immune checkpoint inhibitor in a patient with metastatic melanoma. An 82 year old male presented with widespread metastatic mucosal melanoma and was initially treated with the CTLA-4 inhibitor ipilimumab but had progression of disease after four doses. He was subsequently treated with the PD-1 inhibitor pembrolizumab and after two doses the patient noted bilateral hearing loss. Otology evaluation was significant for the development of bilateral sensorineural hearing loss and the patient was started on treatment with bilateral intratympanic dexamethasone injections. He experienced significant recovery of his hearing deficit with the intratympanic injections and restaging imaging after 12 weeks of pembrolizumab demonstrated a dramatic reduction in tumor burden. Autoimmune inner ear disease has been previously associated with the therapeutic transfer of genetically engineered lymphocytes as an on-target effect of donor T-cells recognizing antigens on cells in the inner ear. It is important for physicians to have a high clinical index of suspicion for the appropriate recognition and management of any potential autoimmune toxicity with checkpoint inhibitors given the variability of presentation and unique aspects of toxicity.
Severe Hyponatremia and Immune Nephritis Following an
Initial Infusion of Nivolumab. Vandiver, Singer, Harshberger. Target Oncol. 2016 Mar 4.
Anti-programmed
cell death-1 (PD-1) antibodies pembrolizumab and nivolumab are becoming increasingly
important in the treatment of melanoma and non-small cell lung cancer. These
agents are known to induce many immune-related adverse events, but rapid-onset
nephritis and immune-related hyponatremia have not been described to date. We
describe the case of an adult patient who developed severe hyponatremia and
rapid-onset nephritis following the first infusion of nivolumab for metastatic
melanoma.
Pembrolizumab-induced necrotic myositis in a patient
with metastatic melanoma. Vallet, Gaillet, Weiss, et al. Ann Oncol. 2016 Mar 2.
Toxic Epidermal Necrolysis-like Reaction With Severe
Satellite Cell Necrosis Associated With Nivolumab in a Patient With Ipilimumab
Refractory Metastatic Melanoma. Nayar, Briscoe, Fernandez
Penas. J Immunother. 2016 Mar 1.
Nivolumab is a fully humanized monoclonal antibody to PD-1, which has shown improved overall and progression-free survival. Across studies of nivo, grade 3 or 4 rash has been noted in more than 1% of patients. We present a case report of a pt with metastatic melanoma...who developed toxic epidermal necrolysis. A 64 year old female presented with widespread maculopapular skin rash with bullae and areas of skin detachment after receiving 2 doses of nivo for ipi refractory metastatic melanoma (BRAF wild type). She was initially treated with prednisone, which was soon changed to methylprednisone followed by immunoglubulin with minimal response to the rash. After discussion with
Dermatology, she was given cyclosporine and high-dose prednisone with gradual
but significant improvement in her rash. Her skin biopsy showed interface
dermatitis with a lymphocytic infiltrate in the dermoepidermal junction and
apoptotic keratinocytes with focal areas of complete necrosis of the epidermis
with minimal infiltrate.
Be sure to talk to your doctor if you think any of these are other side effects may be happening to you!!!! - c
Sunday, February 11, 2018
For Jubes...and the rest of us!!! An anti-rheumatic drug that increases the effect of vemurafenib and selumetinib????
The minute B found this article he was yelling, "Oh, this might be important to Jubes!!!" Well, I hope not!!! Many (actually - probably most!!!) folks who take immunotherapy deal with some amount of arthralgias (joint pain). However, my sweet Anne-Louise dealt with significant arthritis/spondylitis due to her immunotherapy treatment for melanoma! However, after prednisone and other meds like infliximab she got her joint pain under control and threw melanoma to the curb!! But....immune disease and side effects with immunotherapy remain a significant double decker problem.
First....what if you already know you have an immune disease like rheumatoid arthritis? Can you still take immunotherapy? The short answer is YES!!! You can!! Here is a recent post on the subject with more links within: Immunotherapy for melanoma with a pre-existing autoimmune disease??? YES!!! You can!
Here's another report: Patients with preexisting immune disease, melanoma, and treatment with Anti-PD-1? Yes, this can be done. Yes, autoimmune flares should be treated with immunosuppressive therapy while on immunotherapy. And YES!!!! These patients can still attain a response!
Second...there are the folks who didn't have arthritis or other autoimmune diseases (that they knew of) at the start of treatment...but end up with all sorts of immune related side effects caused by their immunotherapy. What should we do with them???? Treat them! Sometimes with a break of their immunotherapy, but with prednisone and other drugs as needed. Right then, right there! And, YES!!! They can still go on to have a response and rid themselves of melanoma in the process! (Just like Jubes!!!) Here are related posts: One more time: Immunosuppressive therapy to manage side effects to immunotherapy does NOT affect response! New report.
And this: Immune related side effects from immunotherapy can and SHOULD be treated!!!!
And this: Side effects and how to manage them in targeted and immunotherapy for melanoma
And finally...this post with a treatment algorithm for neuro side effects and links to algorithms for other immune related side effects: Neurologic side effects to immunotherapy with treatment algorithm
Okay! You'd probably like me to get to a point! What if there was a drug that helped folks with their rheumatic disease AND helped get rid of their melanoma???? Well now, there's this:
The anti-rheumatic drug, leflunomide, synergizes with MEK inhibition to suppress melanoma growth. Hanson, Robinson, Al-Yousuf, et al. Oncotarget. 2017 Dec 17.
Cutaneous melanoma, which develops from the pigment producing cells called melanocytes, is the most deadly form of skin cancer. Unlike the majority of other cancers, the incidence rates of melanoma are still on the rise and the treatment options currently available are being hindered by resistance, limited response rates and adverse toxicity. We have previously shown that an FDA approved drug leflunomide, used for rheumatoid arthritis (RA), also holds potential therapeutic value in treating melanoma especially if used in combination with the mutant BRAF inhibitor, vemurafenib. We have further characterized the function of leflunomide and show that the drug reduces the number of viable cells in both wild-type and BRAFV600E mutant melanoma cell lines. Further experiments have revealed leflunomide reduces cell proliferation and causes cells to arrest in G1 of the cell cycle. Cell death assays show leflunomide causes apoptosis at treatment concentrations of 25 and 50 µM. To determine if leflunomide could be used combinatorialy with other anti-melanoma drugs, it was tested in combination with the MEK inhibitor, selumetinib. This combination showed a synergistic effect in the cell lines tested. This drug combination led to an enhanced decrease in tumor size when tested in vivo compared to either drug alone, demonstrating its potential as a novel combinatorial therapy for melanoma.
In this study, researchers say that leflunomide...a drug used to treat rheumatoid arthritis...seemed to have "therapeutic value" when it was combined with vemurafenib (a BRAF inhibitor) as well as when it was combined with selumetinib (a MEK inhibitor)...in both BRAF positive and BRAF wild type patients! So far, it seems that this is in petri dishes and real live furry ratties. However, if this works this could be super cool for getting rid of melanoma as well as a boon for folks already dealing with arthritis...or perhaps debilitated by arthralgias due to their melanoma treatment.
It's got a ways to go before we can get too excited. But, I like the direction!!! As for my dear Anne-Louise....I hope that she NEVER needs another melanoma or arthritis treatment....EVER!!!! And...I do hope to hear her play her fiddle someday!!!
Hang in there, ratties! (Those that are furry and those who are not so much!) - c
Sunday, June 24, 2018
Art, information and hope!!! ~ Sketching My Way Through Metastatic Melanoma - by Eleanor Segal
Gotta say! Folks with melanoma are some of the most amazing peeps I have ever known!! I am blessed to have come to know so many incredible melanoma friends. I am very lucky to have Ellie as a dear one for some time. Not only is she a melanoma superhero, she is a talented artist, and author!!!
Ellie has certainly paid her melanoma dues, from a primary on her thigh in 1989 to abdominal mets in 2013 followed by surgeries, a zillion scans, IL-2. Yervoy, Opdivo, BRAF/MEK, side effects...AND....DRUM ROLL PLEASE ~ NED per last scans in May of 2018!!! Those results being well maintained on a Tafinlar/Mekinist combo and low dose prednisone! Way to gut it out, sister!!!
In her book, Sketching My Way Through Metastatic Melanoma, Ellie shares her experiences through words and pictures. She made me smile, feel her worry, and recognize her world as she poignantly shares so much of what we melanoma peeps undergo. She also informs - with excellent reports on the way her therapies worked, were administered, and how she dealt with side effects.
Here are some of my favorite pics (comments are mine):
 |
| Packing is serious, yo!!! |
 |
| As is squaring our shoulders and packing away our fears! Ipilimumab/Yervoy. |
 |
| ONO4538, MDX1106, BMS 936558, Nivolumab, Opdivo! |
 |
| The wait!!!! We've all done plenty of that one, right??? |
 |
| BRAF/MEK, targeted therapy. |
Sketching Her Way Through Metastatic Melanoma
Nearly every melanoma patient or survivor that AIM has met has a coping mechanism, hobby, or pastime—something they do to help them in one way or another through their cancer. The most common one we hear is connecting with other patients through social media. But others include gardening, cooking, writing, and doing yoga.
Eleanor Segal sketches. Ellie is 63, married, and a resident of Portland, Oregon. Ellie always made things, and she went to art school. She didn’t draw consistently through her life; she did metalsmithing, created lotions and potions, and worked in other media. But she began drawing again just a few years ago, and now she has sketched her way through metastatic melanoma.
But let’s go back to the beginning. In 1989, Ellie had a melanoma removed from her right thigh. All was fine until 2013, when her doctor felt something unusual during a routine pelvic exam, which turned out to be fist-sized lymph node full of melanoma. After being diagnosed with metastatic disease, she’s had four surgeries, a month of radiation, three types of immunotherapy, and currently, targeted therapy.
After her metastatic diagnosis, she joined the Portland Chapter of Urban Sketchers, a global organization. Urban sketchers always carry their gear, draw wherever they are from observation, and share their work online. The goal is not to be a perfectionist—not to spend too much time on any one sketch—but to show the world what they see, one sketch at a time.
So Ellie brought her sketchbook with her to all of her appointments, and she began sketching everything around her, from her Interleukin-2 IV fluid bags to the contents of her hospital suitcase to the view out her hospital window.

“I didn’t do this for therapy, or to help other people,” she says. “It’s for me. I make collages, write questions, make lists. Sketching allows me to observe, process, sort things, and navigate complex decisions.” Indeed, many of her sketches are combinations of items in the hospital—such as a scale or a pill—combined with details about treatment. While some patients might take notes, Ellie sketches.
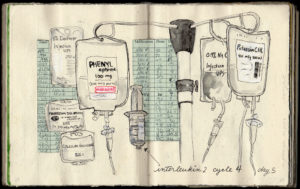
It wasn’t originally a goal to make a book, but she has published a beautiful volume of watercolor sketches entitled “Sketching My Way Through Metastatic Melanoma.” Her family, friends, and healthcare providers are the lucky recipients of her books, and she has kindly included an AIM bookmark inside each volume, reminding those who receive the books to give to melanoma research.
Thank you, Ellie, for capturing in a beautiful form what so many of us have been through, and for letting AIM share a few of your sketches.
You can reach Eleanor Segal via email: billellie@comcast.net
Thursday, August 20, 2015
Immune related side effects from immunotherapy can and SHOULD be treated!!!!
Immune-Related Adverse Events, Need for Systemic
Immunosuppression, and Effects on Survival and Time to Treatment Failure in
Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering
Cancer Center. Horvat,
Adel, Dang, et al. J Clin Oncol. 2015 Aug 17.
Ipilimumab is a standard treatment
for metastatic melanoma, but immune-related adverse events (irAEs) are common
and can be severe. We reviewed our large, contemporary experience with
ipilimumab treatment outside of clinical trials to determine the frequency of
use of systemic corticosteroid or anti-tumor necrosis factor α (anti-TNFα)
therapy and the effect of these therapies on overall survival (OS) and time to
treatment failure (TTF).
We reviewed retrospectively the
medical records of patients with melanoma who had received treatment between
April 2011 and July 2013 with ipilimumab at the standard dose of 3 mg/kg. We
collected data on patient demographics, previous and subsequent treatments,
number of ipilimumab doses, irAEs and how they were treated, and overall
survival.
Of the 298 patients, 254 (85%)
experienced an irAE of any grade. Fifty-six patients (19%) discontinued therapy
because of an irAE, most commonly diarrhea. Overall, 103 patients (35%)
required systemic corticosteroid treatment for an irAE; 29 (10%) also required
anti-TNFα therapy. Defining TTF as either starting a new treatment or death,
estimated median TTF was 5.7 months. Twelve percent of patients experienced
long-term disease control without receiving additional antimelanoma therapy. OS
and TTF were not affected by the occurrence of irAEs or the need for systemic
corticosteroids.
IrAEs are common in patients treated
with ipilimumab. In our experience, approximately one-third of
ipilimumab-treated patients required systemic corticosteroids, and almost
one-third of those required further immune suppression with anti-TNFα therapy. Practitioners and patients should be
prepared to treat irAEs and should understand that such treatment does not
affect OS or TTF.
Pancreatitis Secondary to Anti-Programmed Death
Receptor 1 Immunotherapy Diagnosed by FDG PET/CT. Alabed,
Aghayev, Van den Abbeele. Clin Nucl Med.
2015 Aug 18.
A 57-year-old man with metastatic melanoma developed colitis
secondary to ipilimumab, a known immune-related adverse event (irAE). The
patient then received pembrolizumab immunotherapy, an
anti-programmed-death-receptor-1 (PD-1) antibody. Restaging FDG PET/CT study
following 3 cycles of therapy demonstrated diffuse increased FDG uptake
throughout the body of the pancreas associated with fat stranding in the
peripancreatic region, suggestive of pembrolizumab-induced pancreatitis.
Although the patient was clinically asymptomatic, diagnosis was biochemically
confirmed with elevated amylase and lipase levels. In the era of immunotherapy,
it will be critical to recognize irAEs early to allow prompt initiation of
appropriate therapy and reduce the risk of long-term sequelae.
I posted this in 2014: Melanoma patients teach us more about complications with ipi
It is becoming more and more clear that the old "bug-a-boo" of, "Oh, you're on anti-PD1 (or ipi)! You can't treat side effects with prednisone or any other immune suppressing medication!!! If you do, the therapy won't work on your melanoma!" IS WRONG!!!!!!!!! Rather, if immune side effects are noted as soon as possible and TREATED, it is much more likely that the melanoma patient can continue their needed treatment (perhaps with a drug holiday for a brief time), still receive benefit from that treatment, and diminish the likelihood of long-term problems from those side effects!
Be vigilant my friends. And, be sure that you are going to a doc who knows how to recognize and TREAT any side effects that may develop. Yours - c
Thursday, July 6, 2017
Side effects of immunotherapy - Part 9
And it continues.... Here is a link to Part 8 as well as prior posts: The Saga of Side effects to Immunotherapy
Now these:
Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: Case series and immunophenotypic analysis. Lomax, McGuire, McNeil, et al. Int J Rheum Dis. 2017 May 8.
Sarcoidosis is a multisystem granulomatous disease. This condition has a documented association with the diagnosis of melanoma and can be induced in melanoma patients receiving anti-neoplastic therapy. We evaluated a case series of melanoma patients who developed immunotherapy-induced sarcoidosis. Three patients with melanoma (n = 1 resected Stage III, n = 2 metastatic) treated with anti-programmed cell death (PD)-1 antibody therapy at two institutions developed biopsy-proven sarcoidosis. We used mass cytometry to determine expression of the relevant chemokine receptors (CR) by peripheral blood mononuclear cells for two of the three patients who developed sarcoidosis and 13 melanoma patients who did not. Blood samples were collected before receiving PD-1 checkpoint inhibitor therapy. Immunophenotypic analysis demonstrated abnormally high numbers of circulating Th17.1...cells prior to commencing PD-1 checkpoint inhibitor therapy in five of 15 melanoma patients, including both the patients who developed sarcoidosis during the course of therapy. Our findings support prior literature implicating Th17.1 cells in the pathogenesis of sarcoidosis. However, we demonstrate these findings in patients with melanoma prior to administration of checkpoint therapy and before the onset of clinically symptomatic sarcoidosis. The identification of elevated Th17.1 cells in melanoma patients who have not developed sarcoidosis may reflect the established association between melanoma and sarcoidosis. With some patients receiving these agents over a prolonged period, the clinical course of immunotherapy-induced sarcoidosis is uncertain.
Inflammatory
Myopathy and Axonal Neuropathy in a Patient With Melanoma Following
Pembrolizumab Treatment. Diamantopoulos,
Tsatsou, Benopoulou, et al. J Immunother. 2017 May 11.
Immune-mediated
adverse effects of immune checkpoint inhibitors are rather common,
but neuromyopathic immune-related adverse events are very rare. In
this report, we present a unique case of a patient with a complex
neuromyopathic syndrome with axonal neuropathy and inflammatory
myopathy after a single dose of pembrolizumab. An 82-year-old patient
with a previously untreated stage IIIc melanoma developed ptosis in
the left eye, generalized weakness, and neck and shoulder pain 15
days after pembrolizumab administration. He had left-sided ptosis and
miosis, with a normal pupillary light reflex, horizontal diplopia,
and voice hoarseness, along with weakness of the neck muscles and a
hypokinetic right vocal cord at laryngoscopy. The laboratory
evaluation was remarkable for the marked increase in the serum
lactate dehydrogenase and creatine phosphokinase levels. Further
evaluation revealed findings compatible with axonal neuropathy and
inflammatory myopathy. The patient was treated with corticosteroids,
immunoglobulin, and plasmapheresis, with a minor response; the
patient eventually died. This case represents a newly described
syndrome probably associated with pembrolizumab administration.
Neurologic
Serious Adverse Events Associated with Nivolumab Plus Ipilimumab or
Nivolumab Alone in Advanced Melanoma, Including a Case Series of
Encephalitis.Larkin,
Chmieloski, Lao, Hodi, Weber, et al. Oncologist. 2017 May 11.
Despite
unprecedented efficacy across multiple tumor types, immune checkpoint
inhibitor therapy is associated with a unique and wide spectrum of
immune-related adverse events (irAEs), including neurologic events
ranging from mild headache to potentially life-threatening
encephalitis. Here, we summarize neurologic irAEs associated with
nivolumab and ipilimumab melanoma treatment, present cases of
treatment-related encephalitis, and provide practical guidance on
diagnosis and management. We
searched a Global Pharmacovigilance and Epidemiology database for
neurologic irAEs reported over an 8-year period in patients with
advanced melanoma receiving nivolumab with or without ipilimumab from
12 studies sponsored by Bristol-Myers Squibb. Serious neurologic
irAEs were reviewed, and relationship to nivolumab or ipilimumab was
assigned. In
our search of 3,763 patients, 35 patients (0.93%) presented with 43
serious neurologic irAEs, including neuropathy (n = 22),
noninfective meningitis (n = 5), encephalitis (n = 6),
neuromuscular disorders (n = 3), and nonspecific adverse
events (n = 7). Study drug was discontinued (n = 20),
interrupted (n = 8), or unchanged (n = 7). Most
neurologic irAEs resolved (26/35 patients; 75%). Overall, median time
to onset was 45 days (range 1-170) and to resolution was 32 days
(2-809+). Median time to onset of encephalitis was 55.5 days (range
18-297); four cases resolved and one was fatal. Both
oncologists and neurologists need to be aware of signs and symptoms
of serious but uncommon neurologic irAEs associated with checkpoint
inhibitors. Prompt diagnosis and management using an established
algorithm are critical to minimize serious complications from these
neurologic irAEs. The Oncologist 2017;22:1-10Implications
for Practice: With increasing use of checkpoint inhibitors in
cancer, practicing oncologists need to be aware of the potential risk
of neurologic immune-related adverse events and be able to provide
prompt treatment of this uncommon, but potentially serious, class of
adverse events. We summarize neurologic adverse events related to
nivolumab alone or in combination with ipilimumab in patients with
advanced melanoma from 12 studies and examine in depth 6 cases of
encephalitis. We also provide input and guidance on the existing
neurologic adverse events management algorithm for nivolumab and
ipilimumab.
Autoimmune
diabetes induced by PD-1 inhibitor-retrospective analysis and
pathogenesis: a case report and literature review. Gauci, Laly,
Vidal-Trecan, et al. Cancer
Immunol Immunother. 2017 Jun 20.
Anti-PD-1
antibody treatment is approved in advanced melanoma and provides
median overall survival over 24 months. The main
treatment-related side effects are immune-related adverse events,
which include rash, pruritus, vitiligo, thyroiditis, diarrhoea,
hepatitis and pneumonitis. We report a case of autoimmune diabetes
related to nivolumab treatment. A 73-year-old man was treated in
second line with nivolumab at 3 mg/kg every two weeks for
metastatic melanoma. At 6 weeks of treatment, he displayed
diabetic ketoacidosis. Nivolumab was withheld 3.5 weeks and
insulin therapy was initiated, enabling a normalization of glycaemia
and the disappearance of symptoms. Laboratory investigations
demonstrated the presence of islet cell autoantibodies, while
C-peptide was undetectable. Retrospective explorations on serum
banked at week 0 and 3 months before the start of nivolumab,
already showed the presence of autoantibodies, but normal insulin,
C-peptide secretion and glycaemia. Partial response was obtained at
month 3, and nivolumab was then resumed at the same dose. The
clinical context and biological investigations before, at and after
nivolumab initiation suggest the autoimmune origin of this diabetes,
most likely induced by anti-PD-1 antibody in a predisposed patient.
The role of PD-1/PD-L1 binding is well known in the pathogenesis of
type 1 diabetes. Therefore, this rare side effect can be expected in
a context of anti-PD-1 treatment. Glycaemia should be monitored
during PD-1/PD-L1 blockade. The presence of autoantibodies before
treatment could identify individuals at risk of developing diabetes,
but systematic titration may not be relevant considering the rarity
of this side effect.
Myasthenia gravis: An emerging
toxicity of immune checkpoint inhibitors. Makarious, Horwood,
Coward. Eur J Cancer. 2017 Jun 27.
The advent of immunotherapy has
heralded a number of significant advances in the treatment of
particular malignancies associated with poor prognosis (melanoma,
non-small-cell lung, renal and head/neck cancers). The success
witnessed with therapeutic agents targeting cytotoxic
T-lymphocyte-associated protein 4, programmed cell death protein 1
and programmed cell death ligand 1 immune checkpoints has inevitably
led to an explosion in their clinical application and the subsequent
recognition of specific toxicity profiles distinct from those long
recognised with chemotherapy. Consequently, as the utility of such
therapies broaden, understanding the nature, timing and management of
these immune-related adverse events (irAEs) becomes increasingly
significant. Although neurological irAEs are considered relatively
rare in comparison with hepatitis, colitis, pneumonitis and
endocrinopathies, one emerging side-effect is myasthenia gravis (MG).
Among the 23 reported cases of immune checkpoint inhibitor-associated
MG, 72.7% were de novo presentations, 18.2% were exacerbations of
pre-existing MG and 9.1% were exacerbations of subclinical MG. The
average onset of symptoms was within 6 weeks (range 2-12 weeks) of
treatment initiation. In addition, there was no consistent
association with elevated acetylcholine antibody titres and the
development of immune checkpoint inhibitor-related MG. Significantly,
there was a 30.4% MG-specific-related mortality, which further
emphasises the importance of early recognition and robust treatment
of this toxicity. In addition to a review of the existing literature,
we present a new case of pembrolizumab-induced MG and provide
insights into the underlying mechanisms of action of this phenomenon.
Pretty crazy stuff can develop as a side effect from immunotherapy. Over 7 years ago, Weber told me, "This stuff is weird!" No kidding. So, as best you can...report any worrying signs or symptoms to your doc as soon as you can. It may not result in completely eradicating the problem, but it could go a long way in curtailing additional damage or save your life! Furthermore, there is good data that rapid treatment with prednisone, sometimes with a break in treatment and sometimes not, can:
1. Bring the problem under control.
2. Often allows a return to therapy.
3. Does NOT adversely impact response to the melanoma treatment!!!!
Here are previously posted reports on how to deal with side effects to immunotherapy:
A discussion by Weber and Agarwala from 2015: Side effects and how to manage them in targeted and immunotherapy for melanoma
From 2016: How to deal with GI, endocrine, hepatic and pulmonary side effects subsequent to anti-PD-1
From 201: Neurologic side effects to immunotherapy with treatment algorithm
Hang in there peeps!!! - c
Pretty crazy stuff can develop as a side effect from immunotherapy. Over 7 years ago, Weber told me, "This stuff is weird!" No kidding. So, as best you can...report any worrying signs or symptoms to your doc as soon as you can. It may not result in completely eradicating the problem, but it could go a long way in curtailing additional damage or save your life! Furthermore, there is good data that rapid treatment with prednisone, sometimes with a break in treatment and sometimes not, can:
1. Bring the problem under control.
2. Often allows a return to therapy.
3. Does NOT adversely impact response to the melanoma treatment!!!!
Here are previously posted reports on how to deal with side effects to immunotherapy:
A discussion by Weber and Agarwala from 2015: Side effects and how to manage them in targeted and immunotherapy for melanoma
From 2016: How to deal with GI, endocrine, hepatic and pulmonary side effects subsequent to anti-PD-1
From 201: Neurologic side effects to immunotherapy with treatment algorithm
Hang in there peeps!!! - c
Thursday, September 24, 2015
Side effects and how to manage them in targeted and immunotherapy for melanoma
A Team Based Approach to Melanoma: Managing Side Effects with Weber and Agarwala
(Late note: since publication of this post, the link above has been taken down. However, the points below are my transcription of their commentary. - c, 2018)
In the link above, Dr. Jeffrey Weber and Dr. Sanjiv Agarwala provide a video discussion, with slides, regarding side effects as well as their treatment for ipi, anti-PD1, and BRAFi. While none of the information provided is terribly "new" it is presented in an understandable manner in the form of a continuing education program for general oncologists and others in the field...from the horse's mouth.
Here are the points that stuck me:
- Most common side effects with anti-PD1 and anti-CTLA4 are: dermatitis, enterocolitis, hepatitis and endocrinopathies.
- Toxicity does not equal response, but there appears to be a weak association.
- Order of appearance of side effects typical in anti-PD1 = skin, gastric, hepatic, pulmonary, endocrine, then renal...with most of these occurring before week 12 of therapy.
- HOWEVER: Both docs note that side effects, new to the patient, CAN occur late as well. Specifically pointing out that patients can sometimes demonstrate the onset of a new side effect AFTER the 4th dose of ipi or after even 18 months out with anti-PD1.
- Lo and behold!!!!! Docs have deemed side effects cumulative!!!!!!!!!!!!!! Meaning they may start off very mild...but can, at times, progress to the point that the patient has to be taken off therapy. Dr. Weber specifically notes that arthralgias and fatigue can certainly develop in this manner.
- Again, the docs note that side effects have a weak association with effect, but folks with lots of side effects may NOT respond, while those experiencing very few side effects may still gain a positive response.
- Steroids SHOULD be used to treat side effects, sooner rather than later. The greater the grade of the side effect, the higher the dose of prednisone needed, with a longer taper. STEROID USE DOES NOT DIMINISH RESPONSE!!!!
- TELL YOUR DOC....if you think you are experiencing a side effect.
- Side effects for BRAF/MEK drugs:
- Vemurafenib: PHOTOSENSITIVITY, liver problems, rash
- Dabrafenib: rash, FEVER
- Trametinib: rash, diarrhea, RETINOPATHY
- As you can see...there are different problems with each.
- BRAF/MEK inhibitor side effects can be addressed via: dose decrease, drug holiday, steroid use, and alternative dosing schedules.
- While the combining of any medications usually leaves the patient at risk for MORE side effects, combining targeted therapies has DECREASED side effects and increased efficacy!
- Pseudoprogression in immunotherapy: Tumors can enlarge after the start of immunotherapy due to the influx of lymphocytes to the lesion. (However, all the data I can find shows that this only happens about 5% of the time.)
- While some patients have a rapid response, others may be slower to respond: "Be patient with the patient."
- PDL1 staining - a subject still up in the air...but: The presence of PDL1 is predictive of a high rate of response to anti-PD1 drugs, but should not be used to decide treatment. A staining test for PDL1 is not currently readily available nor consistent with the results provided.
- Data shows that patients with a NEGATIVE PDL1 stain, can still attain a 20% response rate to anti-PD1 therapy. Patients with a POSITIVE PDL1 stain are demonstrating response rates greater than 40%.
Thursday, April 29, 2010
Bags are packed and I'm ready to go...
...I guess! Got a few errands done today. Rosie and Momma say I look Chinese with my eyes swollen...I think I just look weird. But all those holes in my head are feeling better. I'm on rather high dose prednisone to decrease any chance of brain swelling after the radiation. Always wanted to be one of those people who clean the house and feel totally jazzed while on it, but I just end up feeling really tired. My sister says I'm normally up running around like a crazy person cleaning the house and stuff so it figures that I would go the other way.
Noticed an interesting occurrence Tuesday: On our way home after the radiation we drove through rain, hail, and sun...some of them all at the same time. We went to the hospital in a horrible snow and ice storm on our way to my first surgery. (I thought we were going to die in a crash before we ever got there.) And on one of my last procedures Bush was in town, and we almost didn't make it to the hospital on time because all the roads were barricaded closed. So, who knows what we will run into tomorrow??!!!!!!
Thanks to all of you for your love and support during all of this. Rosie will be taking over the blog for now.
Looking forward to talking to you more later. You all know I don't like to be still or quiet. Love to all. C
Noticed an interesting occurrence Tuesday: On our way home after the radiation we drove through rain, hail, and sun...some of them all at the same time. We went to the hospital in a horrible snow and ice storm on our way to my first surgery. (I thought we were going to die in a crash before we ever got there.) And on one of my last procedures Bush was in town, and we almost didn't make it to the hospital on time because all the roads were barricaded closed. So, who knows what we will run into tomorrow??!!!!!!
Thanks to all of you for your love and support during all of this. Rosie will be taking over the blog for now.
Looking forward to talking to you more later. You all know I don't like to be still or quiet. Love to all. C
Saturday, December 26, 2015
Yep! Immunotherapy can work in the brain...and pseudoprogression can be real!!
Melanoma brain metastasis pseudoprogression after
pembrolizumab treatment. Cohen, Alomari, Vortmeyer, et
al. Cancer Immunol Res. 2015 Dec 23
"The role of immunotherapy in treatment of brain metastases
is unknown since most trials exclude patients with active brain lesions. As new
immunomodulating agents gain approval for many malignancies, it is important to
know if they have unique effects in the central nervous system (CNS). Here we
present a case of a patient with progressing brain metastases treated with a
single cycle of pembrolizumab, who presented with mental status changes 11 days
thereafter. MRI of the brain showed enlargement of CNS lesions with intense
central enhancement and diffuse perilesional edema. Histologic evaluation of a
resected lesion revealed isolated clusters of tumor cells surrounded by
reactive astrocytosis, scattered inflammatory cells and an abundance of
microglial cells. Given the increasing use of immune checkpoint inhibitors in
patients with brain metastases from melanoma and other diseases, recognition of
pseudoprogression and management with immune suppression is essential."
Y'all know I've been yelling this for awhile:
1. Yes, Virginia....immunotherapy works in the brain. Anti-pd1 in melanoma: It works in the t-cells, brain, and everywhere else!
2. It works even better combined with radiation. Radiation for melanoma: better when combined with immunotherapy
3. No, we didn't allow folks with brain mets in treatments that could help soon enough!
4. Pseuodoprogression is real and it, along with other side effects, SHOULD be treated...even with prednisone (and other immune suppressing drugs) if needed when on immunotherapy!!!!!! Those treatments do NOT impede response, but they can save your life and/or allow you to continue the therapy that will!!!
Whew! Ok. Rant over. I feel better. Now...let's get lots of other folks feeling better. We KNOW this stuff now!! Let's USE what the ratties, who put their lives on the line, taught us for the good of ALL peeps dealing with melanoma in the brain and elsewhere. - c
Subscribe to:
Posts (Atom)